Hemerion Therapeutics has completed a seed round of €3.55 million, which included investment funds, business angels, and a loan from Bpifrance. This fresh round of funding expedites the start of worldwide clinical studies to validate Hemerion’s therapeutic solution for the treatment of glioblastoma, the most aggressive kind of brain cancer.
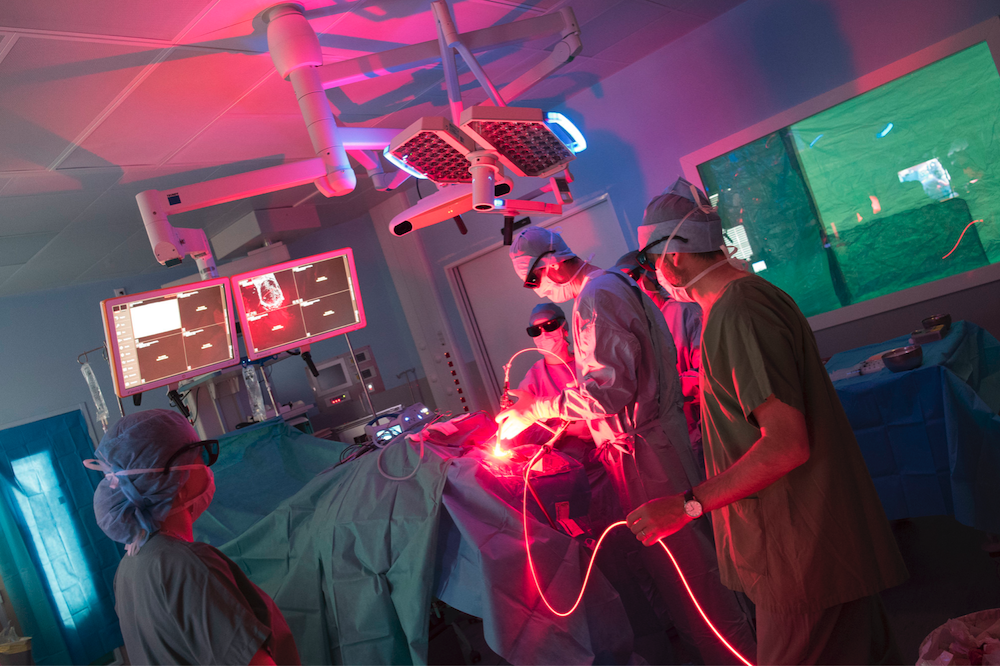
Hemerion’s method combines chemicals with cutting-edge laser treatment to specifically destroy cancer cells while leaving healthy tissue alone. The method is based on a medication that accumulates specifically in cancer cells. When exposed to a certain laser light, the medication kills tumour cells via a photochemical cytotoxic action. Tumor cells are destroyed wherever light enters.
Hemerion technology, which is used as an add-on to surgery before any adjuvant treatments, destroys cancer cells in areas that are unreachable to traditional tumour excision. It is especially promising for the treatment of glioblastoma, one of the most frequent and aggressive brain cancers. Hemerion Therapeutics has already begun its clinical programme with a first definitive clinical trial in glioblastoma patients. Its incorporation into the standard of treatment could increase patient survival and quality of life dramatically.
A significant amount of seed funding that contributes directly to worldwide clinical development.
This significant funding, totaling €3.55 million, provides exceptional development opportunities for Hemerion’s founders and personnel. The FIRA NORD EST 2 (Finovam management), CapTech Santé Nutrition, and Nord France Amorçage funds, as well as several business angels, invested a total of €2.55 million in this round. These equity investments are accompanied by a €1 million startup credit from Bpifrance.
This fresh round of funding comes on the heels of the first round, which was completed by Hemerion’s creators, the University of Lille Foundation, Bpifrance, and a French business angel entrepreneur. Since the company’s inception in September 2020, Hemerion has raised a total of more than €1.7M in investment.
This new amount of funding is set to significantly strengthen the company’s regulatory, clinical, and industrial resources, preparing it for a Series A round in 2023.